Technology
2025 Medical Device Prototyping Guidelines: Avoid 70% Compliance Delays and Accelerate Time to Market
Published
1 hour agoon
By
IQnewswire
Introduction
The medical device sector is characterized by innovation; however, entering this sector with a new innovation is full of challenges. The time taken for developing prototypes is extremely long, and because of this complex matrix of regulatory requirements, delays and cost overruns for new products are tremendous in nature. The main problem often lies with the traditional method of developing prototypes, which considers regulatory integration as a secondary step. If ISO standards are ignored during this stage, it is bound to result in redesign failures and related testing failures as well.
This topic shall be addressed further in this article by providing a means for incorporating precision engineering and rapid prototyping techniques that integrate the regulatory framework right from the onset for a winning business start and enduring business growth.In the subsequent sections, a close analysis shall be performed on how this integrated technique could be implemented for optimal results.
Why Medical Device Prototype Development Must Integrate Regulatory Standards like ISO 13485?
The creation of a prototype of a medical device is an important step in a project’s success or failure. It is an imperative requirement that regulatory requirements such as ISO 13485 must be incorporated at this point and in all of a business’s operations. It is an internationally recognized standard providing requirements for a quality management system particular to medical devices. Biological safety and traceability must be given meticulous consideration at this point.
The process of building a prototype without such a compliance basis is not a prudent undertaking. Flaws in biocompatibility or design history tracing could mean going back to the drawing board, leading to monumental delays and cost escalation. However, a prototype created on a basis that is compliant with ISO 13485 enables a validated process to a marketplace-ready deliverable. By proactively dealing with failure points, there is an improvement in the designs’ reliability. The presence of certifications in a company reflects a concern for quality that fosters trust in business relationships because there are inherent risks that are thus being averted.
How to Select Rapid Prototyping Technology to Accelerate Medical Device Development?
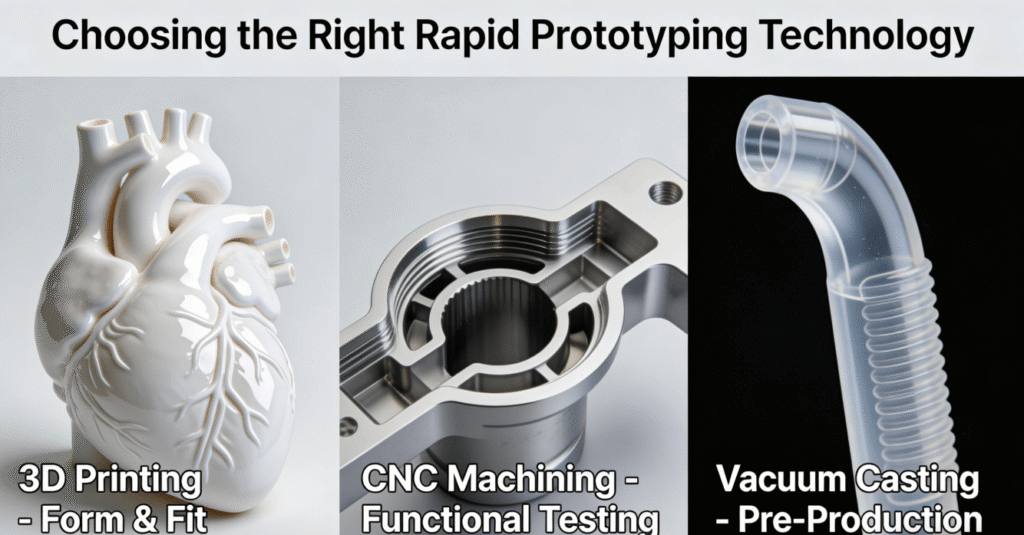
The choice of proper technology for rapid prototyping medical devices is highly important to achieve a balance between speed, cost, and functionality. Highly dependent on the purpose for which the prototype is to be used, whether for modeling, testing, or validation, awareness about the advantages and disadvantages of each technology will play a very important role in successful medical device prototyping and innovation.
Matching Technology to Prototype Purpose
The process of selection should begin with the question that the prototype is supposed to answer. For primary form and fit verification, the speed and cost-effectiveness associated with 3D printing cannot be matched. For functional testing or for simulating the mechanical properties of the end product in the prototype, CNC machining has better strength properties. For a small series of components with properties close to molded production items produced using injection molding, vacuum casting is very appropriate.
The Critical Role of Surface Finish
The surface finish of the prototype is not just a matter of aesthetics; in fact, it is highly functional in healthcare. Surface finish is an essential criterion for biocompatibility, as otherwise the surface may allow the growth of bacteria. And it is also an important consideration with regard to the function of components such as seals or moving components.
- Surface Finish Metrics Definition:
As discussed for Surface Finish, the roughness average measurement (Ra) is an easily measurable metric that must be defined. - Impact on Biocompatibility and Cleaning:
A smooth finish is a crucial element in order to inhibit bacterial colonization on the device and facilitate the possibility of device sterilization. This forms a core component for the medical device prototyping guide. - Functionality Assurance:
A finish matters in components such as fluid paths or sealing surfaces, ensuring functionality or accuracy in their operations, among other functions.
Material Selection for Sterilization and Performance
In addition to the process of manufacturing, material selection plays a key role here. The prototypes developed should ideally be made from materials and processes that can withstand certain processes of sterilization without compromising their integrity and usability by breaking down due to the process and sterilization agents used. In addition to these aspects, the material should also provide sufficient mechanical properties for testing.
How Does Precision Engineering Improve the Reliability of Medical Prototype Designs?
Precision engineering is the task of bringing a design idea from concept to trustworthy prototype with a high fidelity. It is a function of the attention to detail in the realization of small tolerance features, micro-machining, as well as a science-based choice of material of construction. In the engineering of medical device prototype design, it is what differentiates a simple model from a foretelling one.
The application of precision engineering is apparent in micro-fluidic channels, surgical instruments scaled down to a miniaturized format, and other sophisticated applications where every micron matters. The application of precision engineering will ensure that this prototype not only resembles the product being developed but will perform in a similar capacity. The selection of materials in precision engineering as a guiding principle has been discussed to go beyond the usual requirements.
The application of precision engineering will include a meticulous process where potential mistakes associated with dimensional inaccuracies for a functional assembly or materials for product durability will be avoided. The manufacturers with precision engineered equipment following strict levels of quality in IATF 16949 standards will offer quality process control associated with industries like the car trade for the development in medical devices where every prototype produced is a reliable process for replication on a production scale of industrial equipment.
Do you know Importance and Implications of Prototype Development for Medical Devices?
The successful development of medical device prototype development proves to be an excellent innovation and growth catalyst for business growth. Because of its ability to considerably reduce the time required for product development, businesses will now be able to: develop and test products repeatedly among their customers and reach the market first.
Accelerating Time-to-Market
Rapid prototyping helps to explore various design options very quickly, thus aiding innovation and making sure that the final product develops in an optimized manner for both user and market. Rapid prototyping helps to de-risk the major investment in terms of mass production.
- Feedback from the Clinical Environment and Users:
The capacity to generate workable prototypes of clinical or initial regulatory filings is invaluable in ensuring that the final product addresses real-world requirements. - Iterative Design Optimization:
A lean process like that which is possible with the Rapid prototyping service will allow the companies to optimize designs quickly by shifting designs based on real-world feedback and capabilities.
Scalability and Market Development Capability
A prototype phase that integrates smoothly with production, whether it is low volume production or high volume production, is altogether a flawless process when accomplished as a result of a prototype that has been designed with production in consideration (design for manufacturing or DFM). Scalability is a highly essential component for meeting market demand and developing business as a result.
For example, LS Manufacturing has assisted several clients in scaling from prototype validation to production and ensuring consistency and quality throughout this period. The entire process, from conceptualization to production on advanced industrial equipment, involves a highly strategic requirement for a prototype phase that is not mere technology anymore.
What are the Key Trends in Medical Device Prototype Manufacturing for 2025?
To look ahead into 2025, some of the emerging trends are expected to reshape and redefine prototype manufacturing for medical devices. Adoption of these trends will play a very important role in helping organizations retain and sustain their competitive advantage. This 2025 medical device prototype guide highlights these emerging trends.
The growth of digital twin technology, where a digital twin of a physical device is created, is underway. This enables a tremendous amount of simulation and testing in a virtual world, foretelling how a product will work before a single physical prototype has been created. Sustainability is also being prioritized, with more focus on designing prototypes from recyclable materials that are bio-based, without sacrificing performance.
Moreover, the inclusion of AI in design tools will make possible the optimization of geometries for various factors such as density, strength, and materials. Faster and more sophisticated CNC machining as well as more advanced 3D printing technologies will continue to advance with higher resolution and more materials being compatible with these technologies. Organizations certified to an advanced level, such as AS9100D, are poised to take advantage of the increasing call for medical prototypes to demand higher degrees of reliability and traceability, utilizing the precision levels of the aerospace industry for medical innovation.
Conclusion
In conclusion, if medical device prototyping is to overcome current challenges, there must be a holistic approach or strategy that brings precision engineering and rapid prototyping in tandem with a proactive regulatory strategy. This approach greatly minimizes risks and provides a foundation for success and innovation.
Ready to expedite your medical device development? Get a customized prototype solution and start your next project today.
Author Biography
The author is the Senior Manufacturing Engineer at LS Manufacturing. With over a decade of specialized experience, he helps medical device startups and established OEMs solve critical challenges in prototype reliability and regulatory compliance. At LS Manufacturing, a company certified to ISO 13485 and IATF 16949 standards.Ready to de-risk your development timeline?
FAQs
Q1: What is Medical Device Prototype Manufacturing?
A: Medical device prototype manufacturing refers to a process where a prototype of a product is developed to test a design, functionality, or compliance. It allows manufacturers to identify any products with potential defects before mass production.
Q2: What are the advantages of Rapid Prototyping technology?
A: The rapid prototyping techniques, such as 3D printing, can lead to shortened development times, rapid iterations in design, and lower costs due to their ability to produce complex shapes with customized requirements suited to medical devices.
Q3: Why is it important to include regulatory standards in prototyping?
A: It is important to state that regulatory requirements, such as the ISO 13485 standard, help the prototypes meet the requirements of biosafety and quality, thus ensuring the product’s success.
Q4: How to select materials for prototype manufacturing?
A: The choice of materials should take into account biocompatibility, sterilization compatibility, and mechanics and should refer to standards like ISO 10993 to ensure that the prototype is compatible with a medical environment.
Q5: What are the implications of prototype manufacturing on business expansion?
A: Effective prototype development with efficiency allows the company to quickly enter the market and thus assuredly generates revenue.

How Vape Flavours Encourage Adults To Quit Harmful Tobacco Products Safely

Unique Interior Solutions for Modern Homes with Salt Tiles

10 Unique Anniversary Band Ideas for Couples Who Want Something Special

Enterprise SMS Infrastructure: How It Works and Why It Still Drives Business Results

How to Optimize Social Ads for Engagement Without Sacrificing Security

2025 Medical Device Prototyping Guidelines: Avoid 70% Compliance Delays and Accelerate Time to Market

Boosting Industrial Equipment Business Growth by 30% Through Smart CNC Material Selection: Avoiding the Cost Trap

Easy participation, stable returns: WPA Hash cloud mining solution helps investors achieve daily profits.

Discover Your Perfect Look: How Free AI Hairstyle Tools and Face Shape Analysis Are Changing Personal Style

Modern Aluminum Door Designs for Homes & Offices

Who Is Marlene Knaus? The Untold Story of Niki Lauda’s First Wife

Curious About JOI Database? Read This First Before You Click Anything

Jacqueline Bernice Mitchell: The Inspiring Story of Jerry Rice’s Ex-Wife

Where Is Barbara Boothe Now? Inside Her Life After Larry Ellison

Should You Use Wooflix in 2025? Honest Review and Best Alternatives

Where Is Noelle Watters Now? Jesse Watters’ Ex-Wife’s Life After Divorce

Alisande Ullman Today: What Happened After Her Divorce from Leslie Nielsen?

Mickey Middleton: The Untold Story of Bryan Cranston’s First Wife

Where Is Tanya Hijazi Now?: All About Rick James’ Former Wife

Wendy Lang: Meet the Therapist Married to Cenk Uygur

How Vape Flavours Encourage Adults To Quit Harmful Tobacco Products Safely

Unique Interior Solutions for Modern Homes with Salt Tiles

10 Unique Anniversary Band Ideas for Couples Who Want Something Special

Enterprise SMS Infrastructure: How It Works and Why It Still Drives Business Results

How to Optimize Social Ads for Engagement Without Sacrificing Security

2025 Medical Device Prototyping Guidelines: Avoid 70% Compliance Delays and Accelerate Time to Market

Boosting Industrial Equipment Business Growth by 30% Through Smart CNC Material Selection: Avoiding the Cost Trap

Easy participation, stable returns: WPA Hash cloud mining solution helps investors achieve daily profits.

Discover Your Perfect Look: How Free AI Hairstyle Tools and Face Shape Analysis Are Changing Personal Style

Modern Aluminum Door Designs for Homes & Offices
Categories
Trending
-

 Celebrity6 months ago
Celebrity6 months agoWho Is Marlene Knaus? The Untold Story of Niki Lauda’s First Wife
-

 Entertainment5 months ago
Entertainment5 months agoCurious About JOI Database? Read This First Before You Click Anything
-

 Celebrity3 months ago
Celebrity3 months agoJacqueline Bernice Mitchell: The Inspiring Story of Jerry Rice’s Ex-Wife
-

 News3 months ago
News3 months agoWhere Is Barbara Boothe Now? Inside Her Life After Larry Ellison
